John Dalton Although a teacher, a specialiser, and an professional on congenital defect, physicist is best known for his pioneering theory of atomism.

He additionally developed strategies to calculate atomic weights and structures and developed the law of partial pressures.
Early Life
Dalton (1766–1844) was born into a modest Quaker family in Cumberland, England, and for many of his life—beginning in his village college at the age of 12—earned his living as a tutor and public lecturer. when teaching for ten years at a Quaker private school in dye, he emotional on to a teaching position within the burgeoning town of Manchester. There he joined the Manchester Literary and Philosophical Society, that provided him with an interesting intellectual atmosphere and laboratory facilities. the primary paper he delivered before the society was on colour blindness, that afflicted him and is typically still known as green-blindness.
Theories of Atomism and therefore the Law of Partial Pressures
Dalton found out his read of atomism by approach of meteorology, within which he was seriously interested for a protracted period: he unbroken daily weather records from 1787 till his death, his 1st book was meteoric Observations (1793), and he scan a series of papers on meteoric topics before the Literary and Philosophical Society between 1799 and 1801.
The papers contained Dalton’s freelance statement of Charles’s law (see Joseph gladiator Gay-Lussac): “all elastic fluids expand a similar amount by heat.” He conjointly processed what he had distinguished in meteoric Observations—that the air isn’t an enormous chemical solvent as Antoine-Laurent Antoine Lavoisier and his followers had thought, however a system, wherever the pressure exerted by every gas during a mixture is freelance of the pressure exerted by the opposite gases, and wherever the entire pressure is that the add of the pressures of every gas. In explaining the law of partial pressures to skeptical chemists of the day—including Humphry Davy—Dalton claimed that the forces of repulsion thought to cause pressure acted solely between atoms of a similar kind which the atoms during a mixture were so totally different in weight and “complexity.”
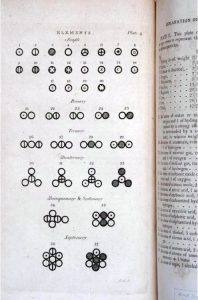
Elements and their mixtures as represented in John Dalton’s New System of Chemical Philosophy (1808–1827).
Elements and their mixtures as represented in John Dalton’s New System of Chemical Philosophy (1808–1827).
Science History Institute
He proceeded to calculate atomic weights from proportion compositions of compounds, victimization AN impulsive system to work out the doubtless atomic structure of every compound. If there square measure 2 components which will mix, their mixtures can occur in an exceedingly set sequence. the primary compound can have one atom of A and one among B; consecutive, one atom of A and 2 atoms of B; consecutive, 2 atoms of A and one among B; and then on. Hence, water is holmium. physicist additionally came to believe that the particles totally different|in several|in numerous} gases had different volumes and surrounds of caloric, therefore explaining why a mix of gases—as within the atmosphere—would not merely layer out however was unbroken in constant motion. physicist consolidated his theories in his New System of Chemical Philosophy (1808–1827).
As a Quaker, physicist semiconductor diode a modest existence, though he received several honors later in life. In Manchester quite forty,000 individuals marched in his ceremonial procession.





